Fire and Truth
Learning from spectroscopy
D. Graham Burnett

Late in the eleventh century, in the thick of the first crusade, when the Christian sackers of Antioch found themselves miserably besieged and starving in the citadel of their prize, a devout soldier of the Lord named Peter Bartholomew, a commoner, awoke from a fitful sleep to announce a vision. Rousing his fellow holy marauders, he led them to a site indicated in his dream and pressed them to dig, whereupon they uncovered a rusty pike, buried deep in the earth. Falling down in ecstatic spasms, Peter declared the relic nothing less than the lance driven into the side of the Savior as he hung upon the cross. Inspired by this apparent sign of divine favor, Peter and the Christians blazed with new vigor, and undertook a breakout maneuver against their infidel enemies, driving them down in a great slaughter.
But not all the knights of Christ were entirely enthusiastic about Peter’s sudden spiritual (and worldly) ascendancy—a certain palpable grumbling could soon be heard in the ranks about this charismatic peasant (whose visions had continued unabated) and his rusty relic (which served him as a kind of scepter). Was he entirely for real? Why not check?
And so, with Peter’s indignant indulgence, a gazebo of dry olive branches was erected, fourteen feet long and four feet high, down the middle of which ran a narrow passage some twelve inches in breadth. After three days of purgatory fasting, Peter emerged from prayerful seclusion dressed in a light shift and carrying his disputed lance. Kindled, the olive bower burst into a tunnel of roaring flame, into which Peter, nearly naked, plunged wildly, disappearing from view in the white heat.
Here, unfortunately, the ancient chroniclers diverge, suggesting a historical record indelibly tainted by partisanship. One line of texts has it that Peter and his false relic were thoroughly consumed in the conflagration, laying permanently to rest his usurpatory deceptions. But a more sympathetic tradition holds that Peter emerged from the fiery gauntlet merely singed, and thus thoroughly exonerated, only to be caught up in the thronging masses of his crazed acolytes, who crushed and trampled him to martyrdom in their enthusiasm—proliferating the immediate supply of relic material, but terminating (despite themselves) his broader bid for temporal leadership.
What is striking in these otherwise incommensurable endings is their perfect consensus on the forensic value of flame: fire, here as elsewhere in the history of the Medieval trial by ordeal, is a basic technology of truth. Burned, the things of the world reveal their essential nature. The scriptural basis for this notion is iffy (Lot surviving the flames of Sodom? Moses’ encounter with the burning bush?). The physics of the proposition, however, proves to be spot-on: everything that burns speaks with tongues of flame that cannot lie. This is called spectroscopy.
• • •
It is itself a phoenix science, at least in its nativist narration. On 10 April 1845, around midday, a small fire broke out in an icehouse on Second Avenue in downtown Pittsburgh, Pennsylvania. In a matter of hours, some fifty acres and more than a thousand structures had been reduced to smoldering embers. As one newspaper reported:
The fire, as though impelled by the hand of the Destroying Angel, rolled on from building to building, with the flight of a fiery flying serpent, consuming every house with the angry fury of a Vulcan, speeding its way with awful and terrific progress, threatening the whole city, inhabitants and all, and only ceased its mad career in the line of the river, because there was nothing more for it to destroy, having swept every thing in its way for one mile and a quarter! Never did any event appear more like Judgment Day. People running, some screaming, others hallowing, warning the people to fly for their lives, carts, drays, furniture wagons, omnibuses, horses, and all and every kind of vehicle, crowded the streets to an excess which made it difficult for each to escape, and threatened destruction to all! May we never again witness such a scene, until the last conflagration of this terrestrial globe!To the ash field of this disaster came, several days later, a thirty-seven-year-old medical doctor and tinkerer named David Alter, resident of the town of Freeport, some two dozen miles northeast of the ruins. The pillar of smoke drew him.
Pittsburgh had been for a generation the center of American glass production, so it was not entirely fortuitous that Alter, in perusing the ruins of the Bakewell-Pears Glasshouse, stumbled on a thick chunk of what looked to be premium flint glass. He pocketed it, and back in Freeport set about grinding it down into a prism. Then, over the next decade, in his small workshop in the Alleghenies, Alter conducted a series of experiments that would earn him footnote status in the history of the physical sciences.
Here is what he did: he rigged up his new prism at the heart of a simple spectroscope, by affixing it in a black box through which light was admitted only via a narrow slit. Pointing this device at a light source—say, the sun—yielded a tidy smear of rainbow that could be projected on a sheet of paper. Alter was well aware that earlier in the century the gifted German optician Joseph von Fraunhofer, messing about with just such an instrument, had noticed that the sun’s spectrum was not perfectly continuous. Rather, it was striped throughout by irregularly spaced dark bars—perhaps ten, perhaps fifty or more (it could be hard to decide, squinting at a blurry rainbow for hours on end; different observers saw things differently). These lacunae in the solar spectrum were a major-minor physics mystery of the first half of the nineteenth century—a challenge to astronomers and a stumper to the rump of natural theologians still trying to read God’s providential inscriptions in the book of nature (the rainbow had been, after all, a divine covenant—why these strike-outs in the contract?).
Like a number of his contemporary practitioners of the natural sciences, Alter was thus keen to investigate these queer lines, and one way to get at the problem was to use his salvage spectroscope to look at a variety of other sources of light: what sort of spectra would they generate? Already equipped with a galvanic generator and other accoutrements of the electromechanical laboratory (he had tried to patent a proto-telegraph several years earlier), Alter rigged up a crankable sparking device which, when juiced and whirling, crackled with a brilliant flash that arced between two metal plates. By swapping out the plates, Alter was able to establish that, although different metals all yielded a more or less “white” spark, this white light, when observed through the spectroscope, resolved not into a full rainbow-like spectrum, but rather into sequences of characteristically colored bars: copper, for instance, consistently produced two orange stripes and three green, together with a bit of fuzzy reddish-yellow; zinc, by contrast, reliably turned up one strong red bar, together with two orange stripes, three of blue, and a little smearing over in the yellow.
Admittedly, this sort of thing had been noticed before, but Alter went so far as to suggest that an alloy like brass (a mixture of copper and zinc) generated a spectrum that superimposed the distinctive color signatures of its component elements. This observation—a little offhand, and published in an American journal not on the desk of every European savant—has earned Alter a claim to be the inventor of “spectral analysis,” the business of reading the tongues of fire.
Put aside his (somewhat tenuous) priority. What matters is that within a decade the notion that each element cannot but declare itself when submitted to the torch would become a central dogma of chemistry, and an extension of this notion to the heavens would mean that every star could be understood to be forever calling out its essential elements. Out of this realization—that starlight was the continuous twinkle of a chorus of celestial confessions—modern astrophysics was born. Even the dark was found to speak: those troublesome privations in the solar spectrum noticed by Fraunhofer were soon shown to correspond to the signature lines of known substances. His lines were, in the end, tell-tale shadows; the materials they signaled were indeed out there, but they were not burning, only lurking in celestial clouds—nevertheless, they could not hide from the pervasive fire of the heavens.
• • •
Alter’s experiments have become standard high school science: a choice laboratory exercise in the modern methods of truth making. And it was with some sense of their canonicity that I set about reproducing them for my sixth-grade science fair. I was, at the time, a basement-dwelling pre-adolescent with an elaborate chemistry set, living in West Philadelphia and attending a forbidding Catholic grammar school. Limited parental oversight had permitted me to install a pump-driven respirator that fed air from the kitchen down to a diving mask I wore when conducting particularly hazardous work with chlorine gas. My assistant, a precocious Mexican neighbor named Dave del Río, had a nervous stutter and no mask. He was very skilled at Dungeons & Dragons.
The spectroscope was easy, but the sparking device never worked properly. Dave and I moved on to a more elaborate scheme: to build a crucible in which we might burn anything—metal salts and even metals themselves. The key lay in an apparatus known as an “arc furnace,” instructions for the building of which we found in a science activity book published before the era of widespread liability litigation. The core of the device involved removing the carbon rod from the center of a pair of D-cell batteries and wiring these pencil-like electrodes to a severed extension cord—one carbon bar attached to one wire, the other to the other. Plugging in the cord, with a well-taped electrode in each hand, one carefully brought their sharpened tips into contact, producing a searing hum, a blinding magnesium-white light—and instantly blowing the mains in the house.
We developed a work-around for this problem: a rheostat to control the current. It was primitive, but very functional: we split one of the electrode leads and wired in a pair of coffee can lids. These were then placed—not touching, weighted down with small stones—in the bottom of a Pyrex baking dish filled with salt water. The water served as a resistor, and by sliding the lids closer together (with a stick, so as not to be electrocuted in the dish of water that was plugged into the wall) we could increase the juice to the arc. Adding more salt also kicked up the charge.
This device worked like a charm. We drilled a pair of holes in a small clay flowerpot, and fed the carbon electrodes through, to make a little crucible. With the arc struck (and carefully maintained by just the right “touch”—the sharpened tips had to be close, but not too close, for maximum heat and light) the pot became a raging inferno in which one could easily and quickly melt down a screwdriver.
Which was entertaining, but we were after bigger game: spectroscopy. So we procured a number of chemical compounds whose emission spectra promised to be clear and bright. My own enthusiasm was highest for some potassium nitrate, which I had found on a dusty back shelf of the local pharmacy, where it was sold, I believe, as an anaphrodisiac (its powers to suppress autoerotic behavior are now discredited). Several heaping tablespoons of this white powder were shoveled into the small furnace, and I struck the arc. Dave stood by with the spectroscope (a shoebox, rigged up with a pinhole and a prism).
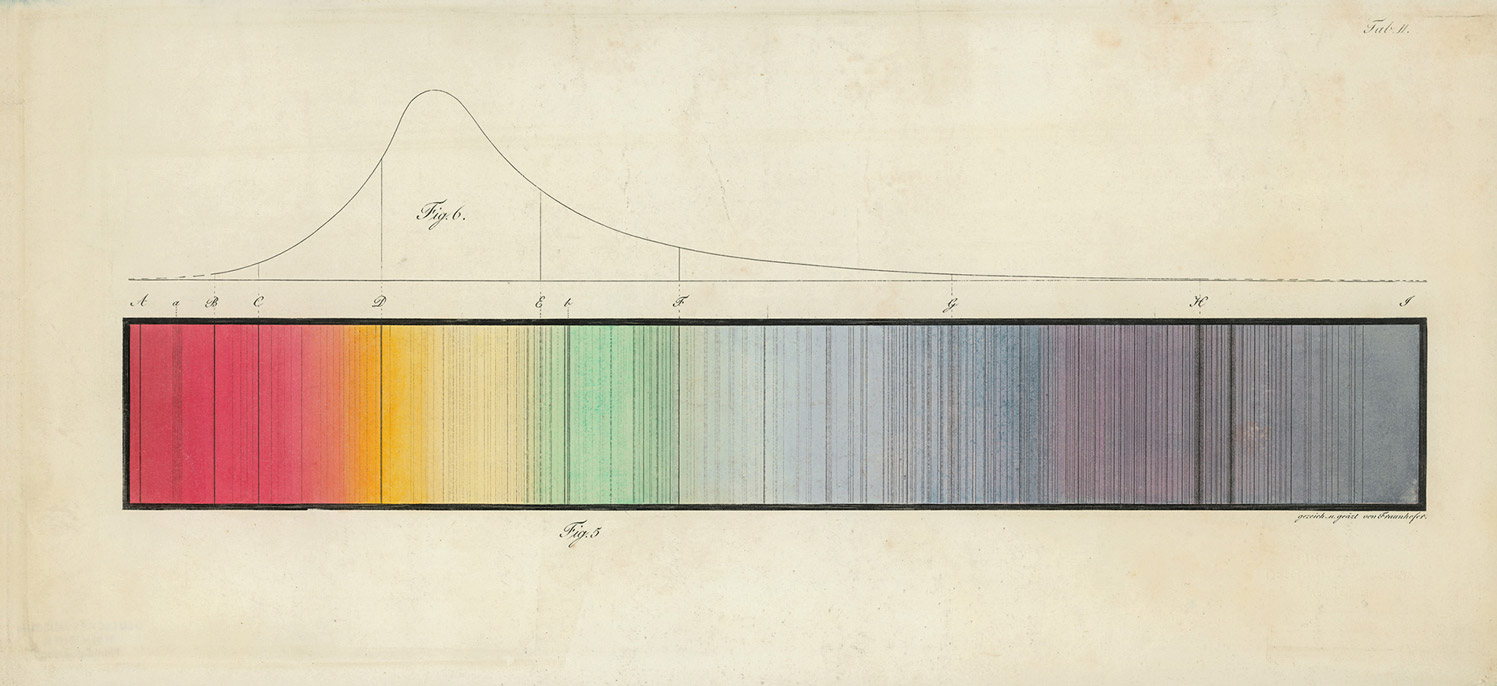
dark lines that would later be read as clues to the chemical composition of extraterrestrial matter. Courtesy Deutsches Museum.
It is difficult to describe what happened next, but it was very scary. I have come to suspect that our potassium nitrate had been adulterated with sugar (perhaps to make it more palatable to little masturbators), which combination—saltpeter and sugar—is the basic recipe for very effective smoke bombs. Whether this was the case or no, the sample, under the influence of the white heat, rapidly congealed into a lava-like ball of orange magma. Immediately unnerved by its spitting and gelatinous vigor, I broke the arc and stepped away, glad to be wearing my respirator. But the cessation of the applied heat did not stop the unfolding autocatalytic process: within seconds, a geyser of furious and terrifying smoke blasted up and choked out the room. Meteoric lumps of molten stone sprayed left and right. Dave, discarding the spectroscope, gasping for breath, upended the rheostat, blowing the fuse and cutting off the flow of air to my mask. Groping, we found our way through the darkness, and tumbled up the steps to the relative safety of the kitchen.
“Did you get a reading?” I asked him, as we lay panting on the floor, still genuinely afraid, thick smoke seeping under the basement door.
“No,” answered Dave. “I couldn’t see a thing.”
• • •
That night I wrote up our results in my laboratory notebook (the basic accoutrement of a science-fair project), and opted to keep the story simple for the nuns: the saltpeter, in my reconstruction, glowed helpfully, and Dave and I were able to witness, at our spectroscopic leisure, a brilliant panorama of the tell-tale emission spectra for both potassium (heavy striping in the blue/violet range together with three nice green bars) and nitrogen (some red, some yellow, some light green). I got all of this, needless to say, from a book. Then I packed up our furnace, and headed off to school for the science fair.
It was in the car that morning that I offered my mother, without too much detail, the story of what had actually happened, and mentioned that I had touched up the results for the consumption of the judges. But she, being at heart a teacher (if not a scientist—she taught French), took the occasion to stump for the virtues of absolute candor in a laboratory notebook. I sat in the back, looking out the window, listening to her expound a general history of the rise of the sciences as nothing less than the sequential progress of unflinching truth over mere slavish orthodoxy. Galileo was brought into evidence; the fortuitous discovery of penicillin made an appearance; disparaging remarks were adduced concerning the anti-empirical tendencies of scholasticism.
I stepped from the car chastened, and resolved to mend my ways. I forfeited recess to the excision of the carefully penned and artfully idealized narrative of our work, and set to scribbling at impassioned speed a faultlessly factual account of the withering disaster: the explosion, the evacuation of the makeshift laboratory, the damage to the fuse-box, my father’s great anger on discovering that the laundry room had sustained fire damage, etc. My “conclusion” section, too, required a total rewrite. No longer the self-satisfied confirmation of the spectroscopic commonplaces, but rather a more provisional and open-ended valediction, calling for further work in this difficult and dangerous area of research.
With a light step, and sensing myself newly a participant in the complex and always contingent process of creating knowledge, I headed down to the gymnasium to set up my arc furnace and spectroscope on a wobbly card-table. I had carefully written out instructions for how the sisters appointed as judges might themselves endeavor to strike the arc, though I decided it was best not to leave any potassium nitrate around. And I did put a sign on the Pyrex baking dish, in large letters, warning everyone to keep their hands out of the water.
• • •
I was, of course, disqualified. Though it entirely surprised me when I returned to my table to find an icy pink disciplinary slip in my (unplugged) crucible, since I had secretly come to expect nothing less than a blue ribbon. Who could have done something better? (The winner, I recall, had looked at his cheek cells under a dime-store microscope.) The nuns had been understandably dismayed by the white-knuckle narrative of the notebook, but more so (and justifiably, it must be said) by the fact that I had left a plugged-in dish of water in a crowded school gymnasium. There would be a reckoning. With Sister William Mary, known among the boys as “Willard, the Rat King.” She who carried (but had not been seen to use) a thick wooden ruler, triangular in section, upon which misfeasors were said to be forced to kneel. It was, of course, a long prism.
Carrying that slip down the carpeted hall of the convent toward the office of the principal that afternoon, I was very afraid: the walls felt close, and I burned with an unhappy combination of shame and anxiety. But at the same time there was, in that small boy’s fear, a delicious shiver of righteousness.
To be a scientist was grand; but to be a martyr—what could compare?
D. Graham Burnett is an editor at Cabinet and a historian of science at Princeton University. He is the author of four books, including Descartes and the Hyperbolic Quest (American Philosophical Society, 2005) and A Trial By Jury (Knopf, 2001). A winner of the 2009 Mellon New Directions Fellowship, Burnett is currently working on the history of aesthetics.
Spotted an error? Email us at corrections at cabinetmagazine dot org.
If you’ve enjoyed the free articles that we offer on our site, please consider subscribing to our nonprofit magazine. You get twelve online issues and unlimited access to all our archives.